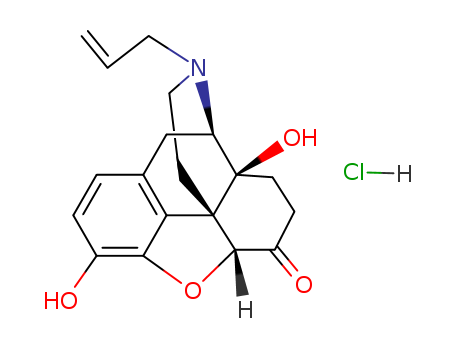
357-08-4
- Product Name:Naloxone hydrochloride
- Molecular Formula:C19H22ClNO4
- Purity:99%
- Molecular Weight:363.841
Product Details
pd_meltingpoint:200-205 °C
Appearance:white to off-white powder
Chinese factory supply Naloxone hydrochloride 357-08-4 in stock with high standard
- Molecular Formula:C19H22ClNO4
- Molecular Weight:363.841
- Appearance/Colour:white to off-white powder
- Vapor Pressure:3.49E-12mmHg at 25°C
- Melting Point:200-205 °C
- Boiling Point:532.8 °C at 760 mmHg
- Flash Point:276.1 °C
- PSA:70.00000
- LogP:2.04130
Naloxone hydrochloride(Cas 357-08-4) Usage
|
Manufacturing Process |
10 grams of 14-hydroxydihydromorphinone (oxymorphone) was converted into its diacetate by warming it on the steam bath with 80 cc of acetic anhydride for about 2 hours. The acetic anhydride was removed on the water bath under a vacuum of about 30 mm absolute pressure. The melting point of the residue was 220°C. The residue was taken up in 100 cc of chloroform. An equal amount by weight of cyanogen bromide was added and the mixture was refluxed at about 60°C for about 5 hours. After refluxing, the mixture was washed with 100 cc of a 5% aqueous hydrochloric acid solution, dried over sodium sulfate and the chloroform removed by evaporation under a vacuum of about 30 mm. The residue had a melting point of 240°C.The residue was then heated at about 90°C for 16 hours on a steam bath with 300 cc of 20% aqueous hydrochloric acid solution, and treated with a small amount, e.g., 1 gram of charcoal. The hydrochloric acid was then removed under a vacuum of 15 mm, the residue dissolved in 30 cc of water and precipitated by the addition of 2.4 cc of concentrated aqueous ammonia. The precipitate was filtered off and dried. It consists of 14- hydroxydihydronormorphinone. It is soluble in ethanol.The 14-hydroxydihydronormorphinone was suspended in 200 cc of pure ethyl alcohol, half its weight of sodium bicarbonate and half its weight of allyl bromide added and the resulting mixture was refluxed at about 75°C for 48 hours. The solution was cooled, e.g., to 10°C and filtered and the alcohol removed under a vacuum of 30 mm. The residue was dissolved in chloroform and filtered. The chloroform was removed under a vacuum of 30 mm and the residue was crystallized from ethylacetate. The crystallized product, N-allyl- 1,4-hydroxydihydronormorphinone, has a melting point of 184°C, is soluble in chloroform and insoluble in petroleum ether. The yield amounts to 20% based on the weight of the reacted 14-hydroxydihydromorphinone. |
|
Therapeutic Function |
Narcotic antagonist |
|
Veterinary Drugs and Treatments |
Naloxone is used in veterinary medicine almost exclusively for its opiate reversal effects, but the drug is being investigated for treating other conditions (e.g., septic, hypovolemic or cardiogenic shock). Naloxone may also be employed as a test drug to see if endogenous opiate blockade will result in diminished tail chasing or other self-mutilating behaviors. It, potentially, could be useful for treating overdoses of clonidine or the CNS effects of benzodiazepines (ivermectin?), but more research is necessary before recommending its use. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Naloxone hydrochloride is rapidly metabolised in the liver, mainly by conjugation with glucuronic acid to naloxone-3-glucuronide, which is excreted in the urine |
|
Definition |
ChEBI: A hydrochloride resulting from the formal reaction of equimolar amounts of naloxone and hydrogen chloride. A specific opioid antagonist, it is used to reverse the effects of opioids, both following their use of opioids during surgery and in cases of known r suspected opioid overdose. |
|
Brand name |
Narcan (Bristol-Myers Squibb); Narcan (Endo). |
InChI:InChI=1/C19H21NO4.ClH/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11;/h2-4,14,17,21,23H,1,5-10H2;1H/t14-,17+,18+,19-;/m1./s1
357-08-4 Relevant articles
Caged Naloxone: Synthesis, Characterization, and Stability of 3- O -(4,5-Dimethoxy-2-nitrophenyl)carboxymethyl Naloxone (CNV-NLX)
Lewin, Anita H.,Fix, Scott E.,Zhong, Desong,Mayer, Louise D.,Burgess, Jason P.,Mascarella, S. Wayne,Reddy, P. Anantha,Seltzman, Herbert H.,Carroll, F. Ivy
, p. 563 - 567 (2018/03/27)
The photolabile analogue of the broad-sp...
Method for synthesis of naloxone hydrochloride
-
Paragraph 0103-0110, (2017/03/22)
The invention belongs to the technical f...
Pharmaceutical combinations of oxycodone and naloxone
-
, (2008/06/13)
Disclosed in certain embodiments is a ph...
Process for the preparation of morphinane derivatives
-
, (2008/06/13)
The invention relates to a process for t...
357-08-4 Process route
-
-
17243-69-5,20380-56-7,20380-58-9,32447-90-8,38690-93-6,51931-66-9,112244-09-4
Tilidine

-
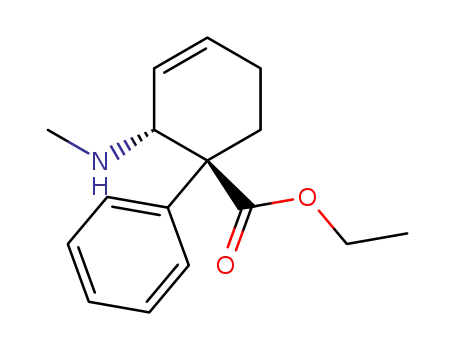
-
34546-23-1,37815-44-4,38677-94-0,67008-95-1,357-08-4
Nortilidine
| Conditions | Yield |
|---|---|
|
With
bromine;
|
-
-
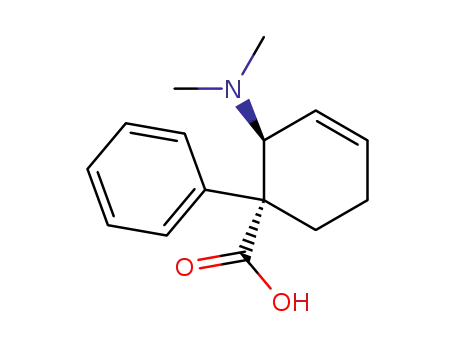
2t-Dimethylamino-1-phenyl-cyclohex-3-en-1r-carbonsaeure

-

-
34546-23-1,37815-44-4,38677-94-0,67008-95-1,357-08-4
Nortilidine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: K2CO3
2: Br2
With
bromine; potassium carbonate;
|
357-08-4 Upstream products
-
124-90-3
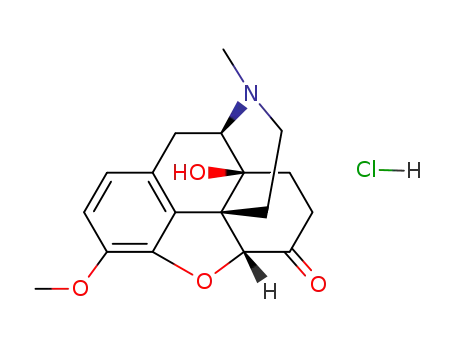
oxycodone hydrochloride
-
115-37-7
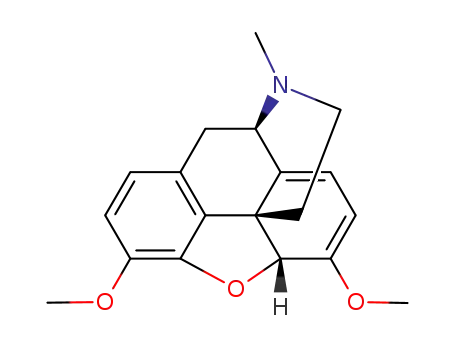
thebaine
-
76-42-6
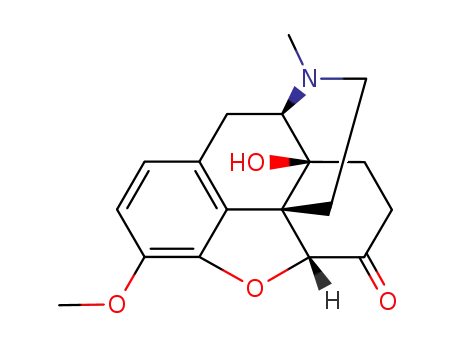
Oxycodone
-
70509-92-1
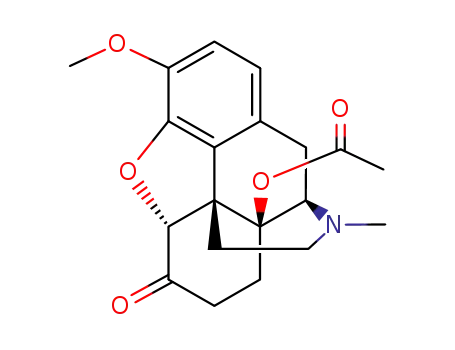
14-acetoxy-4,5α-epoxy-3-methoxy-17-methylmorphinan-6-one
357-08-4 Downstream products
-
34707-87-4
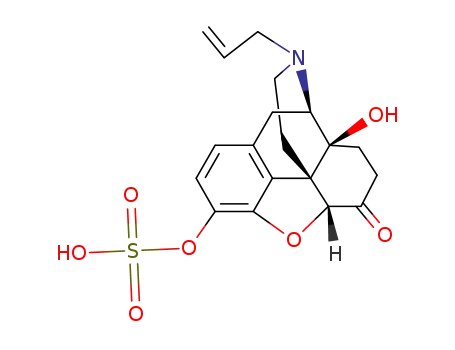
Naloxon-3-sulfat
-
1354744-91-4
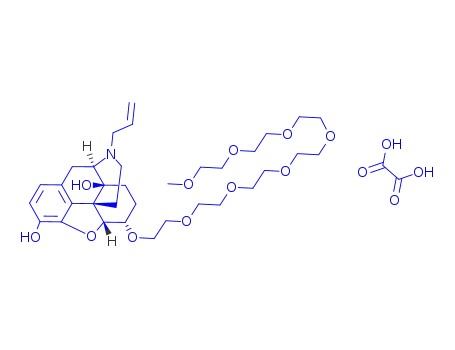
naloxegol oxalate
-
854601-70-0
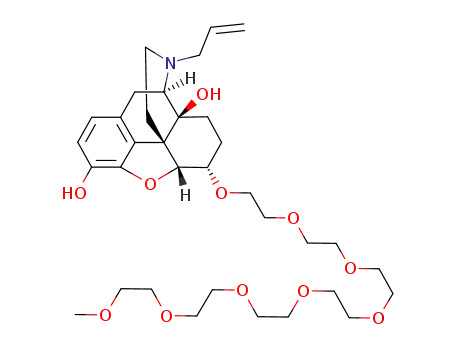
(5α,6α)-17-allyl-6-(2,5,8,11,14,17,20-heptaoxadocosan-22-yloxy)-4,5-epoxymorphinan-3,14-diol
-
16617-07-5
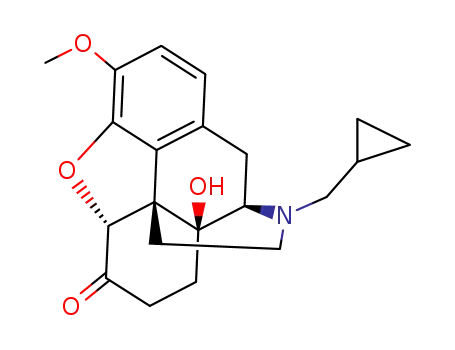
naltrexone methyl ether
Relevant Products
-
Nicotinamide riboside chloride
CAS:23111-00-4
-
Ivermectin
CAS:70288-86-7
-
Minoxidil
CAS:38304-91-5