870-63-3
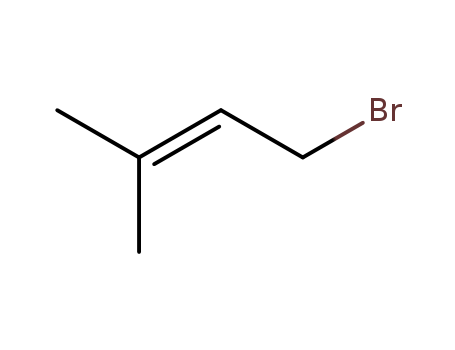
- Product Name:3,3-Dimethylallyl bromide
- Molecular Formula:C5H9Br
- Purity:99%
- Molecular Weight:149.03
Product Details
pd_meltingpoint:-106.7°C (estimate)
Appearance:Light brown liquid with strong pungent odor
Factory sells 3,3-Dimethylallyl bromide 870-63-3 with sufficient production capacity
- Molecular Formula:C5H9Br
- Molecular Weight:149.03
- Appearance/Colour:Light brown liquid with strong pungent odor
- Vapor Pressure:10.8mmHg at 25°C
- Melting Point:-106.7°C (estimate)
- Refractive Index:n20/D 1.489(lit.)
- Boiling Point:82-83 °C (150 mmHg)
- Flash Point:32 °C
- PSA:0.00000
- Density:1.27 g/cm3
- LogP:2.34750
3,3-Dimethylallyl bromide(Cas 870-63-3) Usage
InChI:InChI=1/C5H9Br/c1-5(2)3-4-6/h3H,4H2,1-2H3
870-63-3 Relevant articles
Facile synthesis of deuterium-labelled geranylgeraniols
Totsuka, Yusuke,Ueda, Shota,Shinada, Tetsuro,Kuzuyama, Tomohisa
, p. 575 - 577 (2015)
Facile and stereoselective syntheses of ...
Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes
Hsiao, Chia-Hung Christine,Lin, Xiaochen,Barney, Rocky J.,Shippy, Rebekah R.,Li, Jin,Vinogradova, Olga,Wiemer, David F.,Wiemer, Andrew J.
, p. 945 - 954 (2014)
Phosphoantigen-sensitive Vγ9Vδ2 T cells ...
-
Karrer et al.
, p. 1287,1290 (1939)
-
Using Terpene Synthase Plasticity in Catalysis: On the Enzymatic Conversion of Synthetic Farnesyl Diphosphate Analogues
Hou, Anwei,Dickschat, Jeroen S.
supporting information, p. 15644 - 15649 (2021/10/04)
Four synthetic farnesyl diphosphate anal...
Gold-Catalyzed Formal Hexadehydro-Diels-Alder/Carboalkoxylation Reaction Cascades
Wang, Hong-Fa,Guo, Lin-Na,Fan, Zhi-Bo,Tang, Tian-Hua,Zi, Weiwei
supporting information, p. 2676 - 2681 (2021/04/12)
A dual gold-catalyzed hexadehydro-Diels-...
A mild method for the replacement of a hydroxyl group by halogen: 3. the dichotomous behavior of α-haloenamines towards allylic and propargylic alcohols
Munyemana, Fran?ois,Patiny, Luc,Ghosez, Léon
, (2021/06/07)
A study of the deoxyhalogenation of ally...
METHODS OF SYNTHESIZING FARNESYL DIBENZODIAZEPINONES
-
Paragraph 0082-0083, (2021/12/08)
The present invention is directed to syn...
870-63-3 Process route
-
-
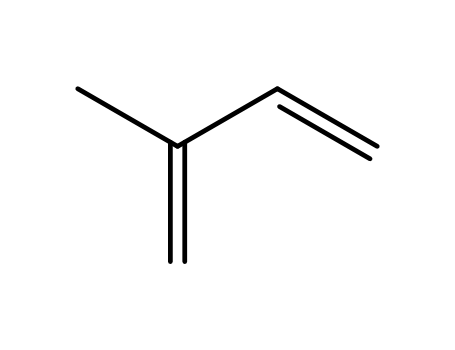
78-79-5,9003-31-0
isoprene

-
-
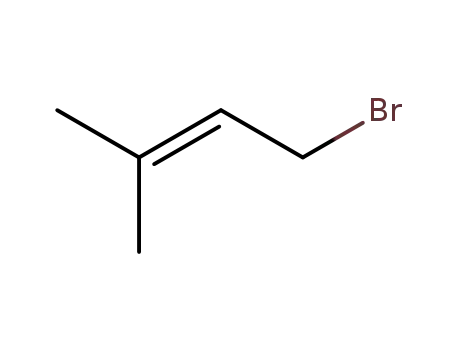
870-63-3
prenyl bromide
| Conditions | Yield |
|---|---|
|
With
phosphorus tribromide;
In
dichloromethane;
at -10 ℃;
for 0.5h;
|
77% |
|
With
silica gel; phosphorus tribromide;
In
dichloromethane;
at -10 ℃;
for 0.416667h;
|
70% |
|
With
hydrogen bromide;
In
acetic acid;
at 0 ℃;
|
64% |
|
With
hydrogen bromide;
In
acetic acid;
at 4 ℃;
for 48h;
|
64% |
|
With
hydrogen bromide; copper(I) bromide;
for 0.166667h;
|
35% |
|
With
hydrogen bromide;
|
|
|
With
hydrogen bromide; acetic acid;
|
|
|
With
hydrogen bromide;
dann Umsetzung des erhaltenen 2-Brom-2-methyl-butens-(3);
|
|
|
With
water; hydrogen bromide; acetic acid;
|
|
|
With
hydrogen bromide;
|
|
|
With
hydrogen bromide; acetic acid;
|
|
|
With
hydrogen bromide;
at 0 ℃;
for 8h;
|
11 g |
|
With
hydrogen bromide;
at 0 ℃;
for 48h;
|
24.5 g |
|
With
hydrogen bromide; copper(I) bromide;
|
-
-
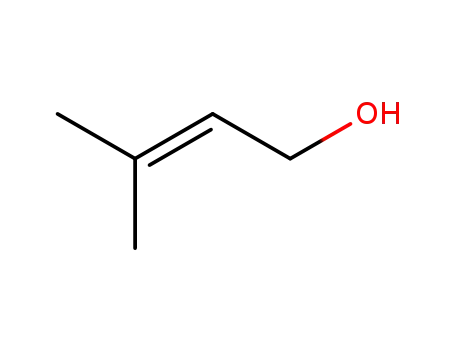
556-82-1
3-methyl-2-buten-1-ol

-

-
870-63-3
prenyl bromide
| Conditions | Yield |
|---|---|
|
With
phosphorus tribromide;
at 0 ℃;
for 30h;
|
95% |
|
With
ziconium(IV) oxychloride octahydrate; sodium bromide;
In
ethanol; water;
at 90 ℃;
for 0.166667h;
|
90% |
|
With
phosphorus tribromide;
|
87% |
|
With
phosphorus tribromide;
In
pentane;
at 4 ℃;
|
85% |
|
With
hydrogen bromide;
In
dichloromethane;
at 0 ℃;
for 1.5h;
|
83% |
|
With
phosphorus tribromide;
In
dichloromethane;
at -20 ℃;
for 3h;
|
82% |
|
With
phosphorus tribromide;
In
dichloromethane;
at -20 ℃;
for 3h;
|
82% |
|
With
pyridine; phosphorus tribromide;
|
80% |
|
With
phosphorus tribromide;
In
diethyl ether;
1.) 0 deg C, 0.5 h, 2.) 25 deg C, 3.) reflux, 1 h;
|
77.5% |
|
With
hydrogen bromide;
In
dichloromethane;
at 0 ℃;
for 2h;
Inert atmosphere;
Darkness;
|
70% |
|
With
phosphorus tribromide;
In
dichloromethane;
at 0 - 20 ℃;
for 1h;
|
62% |
|
With
hexabromoacetone; triphenylphosphine;
In
sulfolane;
|
40% |
|
With
hydrogen bromide;
at 130 - 140 ℃;
|
|
|
With
pyridine; phosphorus tribromide; Petroleum ether;
|
|
|
With
phosphorus tribromide;
In
Petroleum ether;
at 0 ℃;
|
|
|
With
phosphorus tribromide;
In
pentane;
at 0 ℃;
for 0.166667h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
for 15h;
|
|
|
With
phosphorus tribromide;
In
pentane;
at 0 ℃;
for 0.666667h;
|
|
|
With
sulfuric acid; hydrogen bromide;
|
|
|
With
phosphorus tribromide;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
|
|
|
With
phosphorus tribromide;
|
|
|
With
phosphorus tribromide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Inert atmosphere;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
With
carbon tetrabromide; triphenylphosphine;
In
dichloromethane;
at 20 ℃;
for 4h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at -15 - 0 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
With
phosphorus tribromide;
In
tetrahydrofuran;
Inert atmosphere;
|
|
|
With
phosphorus tribromide;
In
dichloromethane;
at 0 ℃;
for 1h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
|
|
|
With
silver(l) oxide;
|
|
|
With
phosphorus tribromide;
at 0 ℃;
for 1h;
Inert atmosphere;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
|
|
With
hydrogen bromide;
at 0 - 20 ℃;
for 1h;
|
60 g |
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 - 20 ℃;
for 7h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 - 20 ℃;
for 16h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
for 1h;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at 0 ℃;
Darkness;
Inert atmosphere;
|
|
|
With
phosphorus tribromide;
In
Dimethyl ether;
at 0 - 20 ℃;
Inert atmosphere;
|
|
|
With
1-chloro-1-(dimethylamino)-2-methyl-1-propene;
In
chloroform-d1;
at 0 - 20 ℃;
for 0.5h;
regioselective reaction;
Inert atmosphere;
|
95 %Spectr. |
|
With
phosphorus tribromide;
In
dichloromethane;
at 0 - 20 ℃;
|
870-63-3 Upstream products
-
556-82-1

3-methyl-2-buten-1-ol
-
115-18-4
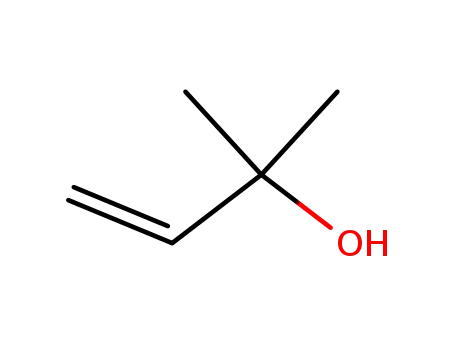
2-methyl-3-buten-2-ol
-
78-79-5

isoprene
-
319-76-6
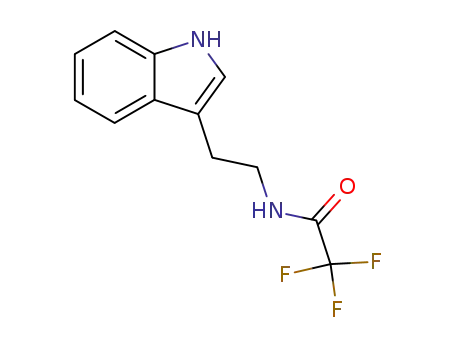
N-(2-(1H-indol-3-yl)ethyl)-2,2,2-trifluoroacetamide
870-63-3 Downstream products
-
4374-93-0
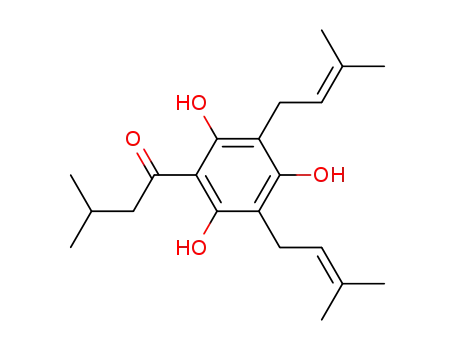
deoxyhumulone
-
54614-64-1
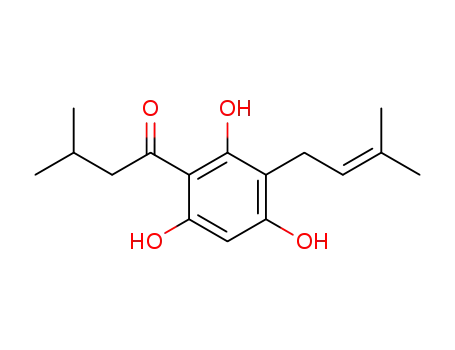
2',4',6'-trihydroxy-3'-(3-methylbut-2-enyl)isovalerophenone
-
94935-91-8
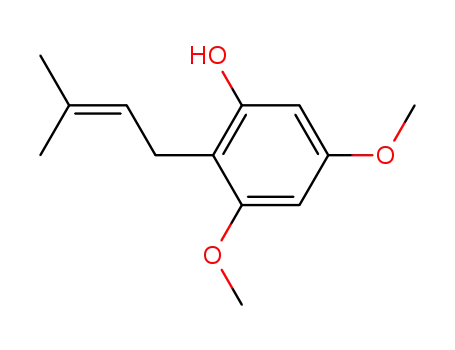
3,5-dimethoxy-2-prenylphenol
-
412340-15-9
5-Benzyl-2,2,7,8-tetramethyl-chroman-6-ol
Relevant Products
-
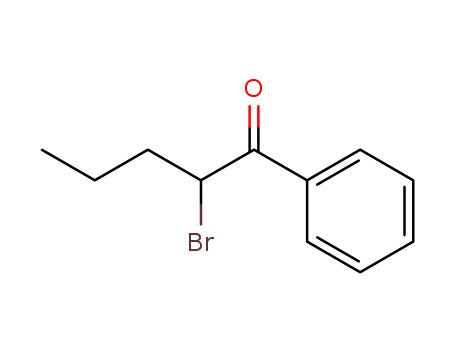
α-Bromovalerophenone
CAS:49851-31-2
-
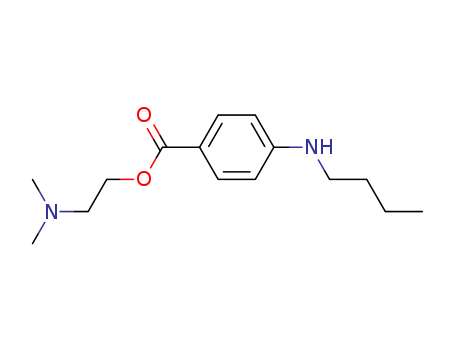
Tetracaine
CAS:94-24-6
-
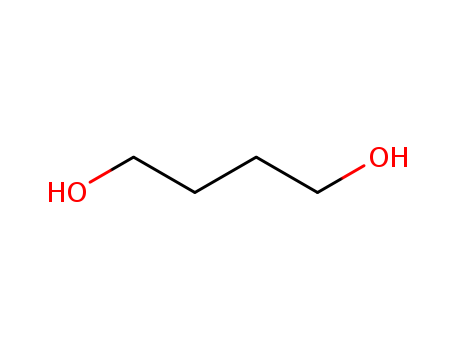
1,4-Butanediol
CAS:110-63-4